
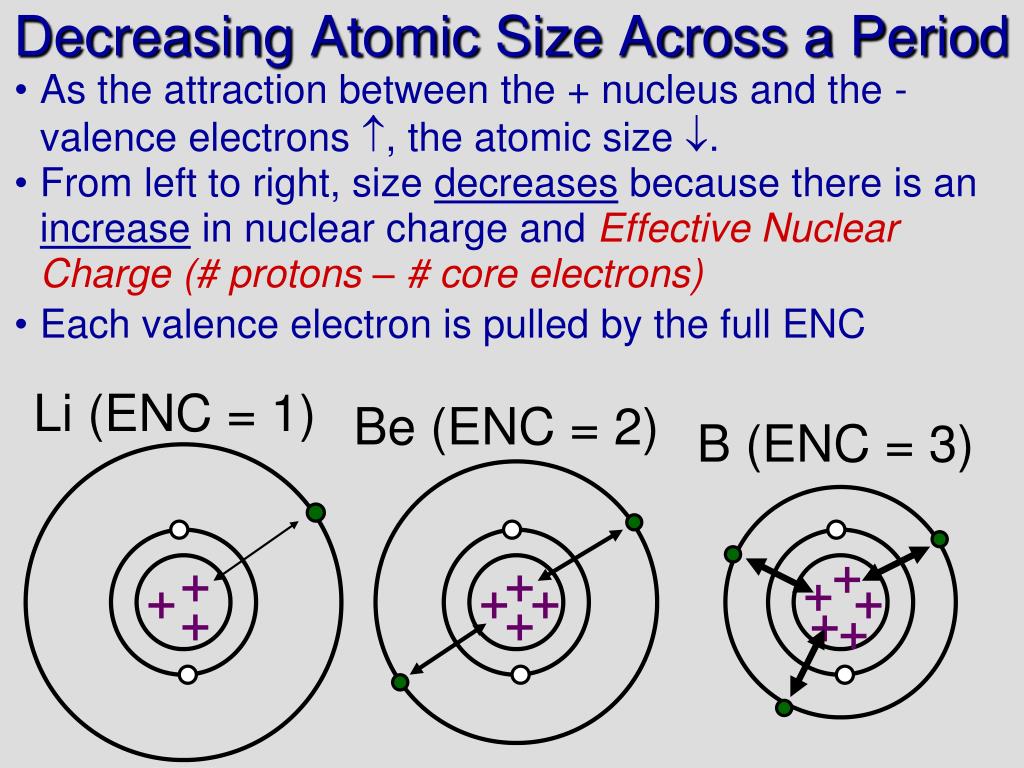
Now the videos stream and the CD seems no longer on offer. The animations in the original were huge for the time (hydrogen was about 12 megabytes), so downloading and saving them was a wise use of bandwidth-or one could buy a CD for $37 (20 pounds). The current version is at least the second. This trend is mainly due to the fact with increase in number. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. Society of Chemistry (UK) are truly exceptional. The atomic size of atoms of elements in a period (d) decreases while going across the period. The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. resourcesīoth the content and design of this site created by the Royal If one thread writes to an atomic object while another thread reads from it, the behavior is well-defined (see memory model for details on data races). Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides.Īcta Crystallographica, Section A. Periodic table of atomic sizes used in molecular models. The atomic radius is used as a measure for the atomic size of the atom, and its measuring unit is picometre (Pm), The picometre is part from million of. The coordination properties and ionic radius of actinium: A 120-year-old enigma.Ĭoordination Chemistry Reviews, vol. In doing so, we found that the atom size seen in TEM images shows a strong correlation to the. (as we move from above to below in a group ) Atomic size decreases as we move from left to right in a period. S is above Te on the periodic table Te is larger because as you go down the column, the atoms get larger. Gauthier J.-P.Deblonde, Mavrik Zavarin, and Annie B. Answer: Atomic size increases with increase in period number. Solution Si is to the left of S on the periodic table it is larger because as you go across the row, the atoms get smaller. From this pattern scientist are able to distance ebetween the atoms and from thst, Elementġ. Also it possesses only one electron in the outermost orbit. I one shines x-rays through the crystel onto a sensor, such as a sheet of photographic film, a pattern of spots. Explanation: Rubidium is a group 1 element and it has bigger atomic size compared to the elements of other groups. The atoms in a crystel are arannged in a regular pattern. They are cfound in clouds.ĭistance beteen teh central charge and the outermost electron. n atom is not like a minature solre sysem either, though it is sometimes helpful to think of it that wat.An atoms electrons don't travil in regualr orbits. We know it has a posoitively charged center (the nucleus), which is surounded by mving electrosn. What does it mean to measure the “size” of an atom?Īn atom is not like a billiard ball.


 0 kommentar(er)
0 kommentar(er)
